The gamma relaxation experiments provided very rich kinetic data. The initial observation was that the rate of polymeriseration is reduced by the addition of the RAFT agent both in in and out of the gamma source. Furthermore, the relaxation from the in-source steady state to the out-of-source steady state was faster in the presence of the RAFT agent [1].
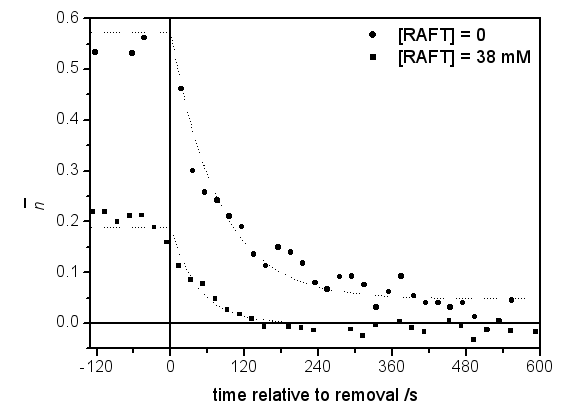
These results may be understood by considering radical loss in RAFT-mediated emulsion polymerisations due to the presence of the RAFT agent (a "RAFT-induced exit" mechanism) along with the chain-length dependence of the termination reaction.
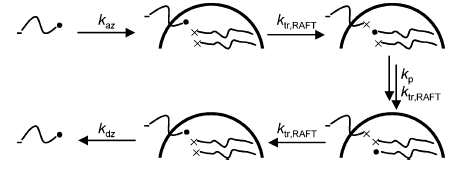
In the case of a RAFT-mediated polymerization, the reaction that can cause the radical to enter the particle can be either propagation or transfer of radical activity to a dormant chain. This reaction leaves a z-meric-RAFT adduct -IMz-S-C(Z)=S on the surface of the particle that is then able to be reactivated and possibly desorb later in the reaction (RAFT-induced exit).
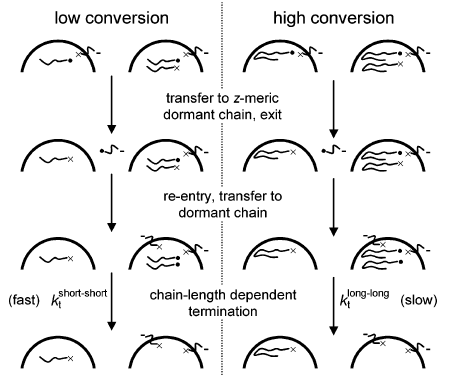
The chain-length dependence of the termination reaction varies as a function of the conversion, as the length of the dormant chains and radicals increases linearly with conversion (see modelling work for details).
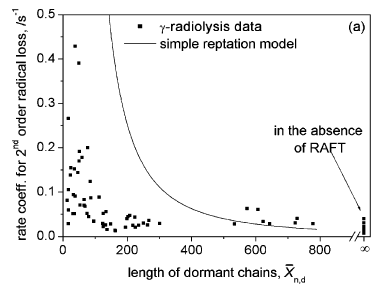
Combining these allows the rate coefficient for second order radical loss to be semi-quantitatively estimated [2].
References
- Radical loss in RAFT-mediated emulsion polymerizations. SW Prescott, MJ Ballard, E Rizzardo and RG Gilbert. Macromolecules, 38(11), 4901–4912, 2005.
- Average termination rate coefficients in emulsion polymerization: effect of compartmentalization on free-radical lifetimes. SW Prescott, MJ Ballard and RG Gilbert. Journal of Polymer Science Part A: Polymer Chemistry, 43(5), 1076–1089, 2005.
Last edited: Tuesday September 9, 2008
Copyright © 1996-2014 Stuart Prescott