Octanol was chosen as an analogue to styrene as the solubility parameter for octanol and styrene is almost identical. The use of octanol rather than styrene allowed the PPPDTA concentration to be directly determined by UV spectroscopy. A standard solution of PPPDTA in octanol was prepared and then five samples spanning two orders of magnitude in concentration (0.3 to 30 mg in 10 mL of octanol) were prepared volumetrically from the standard solution.
Aliquots of PPPDTA in octanol (10 mL) and milliQ water (10 mL) were prepared and placed on a shaker-table for 48 hours. The octanol and water layers were separated using a separating funnel. The absorbance of an intense chromophore in the UV region was measured for each of the octanol and aqueous samples. When necessary, the samples were diluted by factors of 10 or 100 to achieve an experimental absorbance of less than 1, and the absorbances scaled accordingly using Beers Law, giving a Beers Law absorbance which will be used from here on.
The rescaled absorbance data are shown in the figure (it should be noted that the measured absorbance was not 102 but 1, at a dilution of 1:100). Interestingly, the amount of chromophore observed in the water phase was found to be a constant fraction of the PPPDTA in the octanol phase. Moreover, the level of chromophore detected in the aqueous phase (0.45% on PPPDTA) was less than the impurity level in the sample (~1%). It therefore seems unreasonable to conclude that this relationship has specific meaning to the PPPDTA.
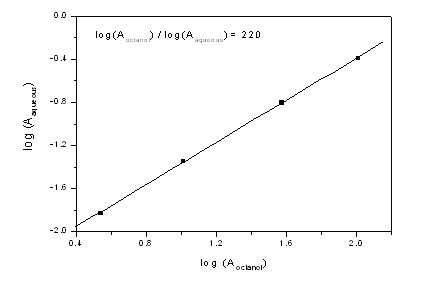
The absorbance in the aqueous phase as a function of the Beers Law absorbance in the octanol phase. The data for the least concentrated sample was discarded, as there was no detectable chromophore in the UV region. The regression coefficient for the linear fit shown above was better than 0.999. Note: experimental absorbance data were between 0 and 1, the reported absorbance was scaled as a function of concentration of the original sample (see text).
It is felt, however, that this partitioning study does indicate some important things. The most direct conclusion that may be drawn is that PPPDTA is highly insoluble in water. This has obvious implications for the transport of PPPDTA from monomer droplets (into which it would partition in an ab initio system) to proto-particles.
Furthermore, these data indicate that water washing may be a suitable way of removing some of the remaining impurities. This is particularly important for emulsion polymerisation systems where contaminants in the water phase can lead to long inhibition or retardation periods. This may offer both an explanation and a solution to the inhibition periods seen in other studies.
Last edited: Wednesday June 22, 2005
Copyright © 1996-2014 Stuart Prescott